2013年3月22日 星期五
遊戲作業:ONE
http://more2.starfall.com/m/math-k/numbers-content/load.htm?f&d=demo&n=num-01&y=1
請問你總共看到多少種東西呢?
請問你總共看到多少種東西呢?
遊戲作業:amazing food detective
http://www.primarygames.com/science/nutrition/games/amazingfooddetective/index.htm
2013年3月19日 星期二
Science for Kids
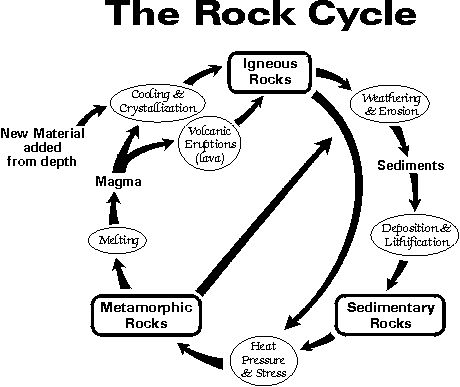
Crystals are a special kind of solid material where the molecules fit together in a repeating pattern. This pattern causes the material to form all sorts of unique shapes. Amethyst Crystal How do they form? The process of crystal forming is called crystallization. Crystals often form in nature when liquids cool and start to harden. Certain molecules in the liquid gather together as they attempt to become stable. They do this in a uniform and repeating pattern that forms the crystal. In nature, crystals can form as liquid rock, called magma, cools. If it cools slowly, then crystals may form. Many valuable crystals such as diamonds, rubies, and emeralds form this way. Another way crystals from is when water evaporates from a mixture. Salt crystals often form as salt water evaporates. What unique properties do crystals have? Crystals can have very flat surfaces called facets. They can form geometric shapes such as triangles, rectangles, and squares. The shapes are a direct result of the type of molecules and atoms that make up the crystal. Smaller crystals and larger crystals that were formed of the same molecules and in the same method should have similar shapes. There are seven basic crystal shapes, also called lattices. They are Cubic, Trigonal, Triclinic, Orthorhombic, Hexagonal, Tetragonal, and Monoclinic. Interesting Types of Crystals Snow flakes - Snow flakes are ice crystals that are formed high in the clouds when water freezes. They always have six sides or arms, but every one of them is unique.  |
Metal Facts
http://chemistry.about.com/od/metalsalloys/a/Metal-Facts-Sheet.htm


This is a crystal of the metal bismuth. The rainbow colors result from a very thin layer of oxidation on the surface of the bismuth.
Alchemist-hp, Creative Commons License Most of the elements in the periodic table are metals. You use metals every day, but how much do you actually know about them? Here is a list of facts and trivia about metals.
- The word 'metal' derives from the Greek word 'metallon,' which means to mine, excavate or extract from the ground.
- 75% of all the elements on the periodic table are metals. The metals are subdivided into separate groups, such as basic metals, transition metals, alkali metals, alkaline earth metals, rare earth, lanthanides and actinides.
- At room temperature, all of the metals are solids except for mercury, which is a liquid.
- The most common metal found in the Earth's crust is aluminum.
- Even though aluminum is abundant in the crust, the most abundant element in the entire Earth is iron, which makes up a large part of the Earth's core.
- Up until Medieval Times, there were only 7 known metals, which were called the Metals of Antiquity. The Metals of Antiquity and their approximate discovery dates are:
- Gold (6000 BC)
- Copper (9000 BC)
- Silver (4000 BC)
- Lead (6400 BC)
- Tin (3000 BC)
- Iron (1500 BC)
- Mercury (1500 BC)
- Most metals are shiny and have a characteristic metallic luster.
- Most metals are good conductors of heat and electricity.
- Many metals are heavy or dense, although some metals, such as lithium, are light enough to float on water!
- Most metals are hard.
- Most metals are malleable or may be beaten into a thin sheet.
- Many metals are ductile or capable of being draw into a wire.
- Many metals are sonorous or make a bell-like sound when struck.
- Metals are elastic or tend to bend rather than break.
- Metals known as metalloids or semimetals have properties of both metals and nonmetals.
- Alkali metals, such as lithium, sodium, potassium, and rubidium, are so reactive they will ignite and even explode if placed in water.
- Despite what you read in books and see in movies, most radioactive materials do not glow in the dark. However, some radioactive metals either glow from internal heat or else release radiation that reacts and produces visible light. Examples of radioactive metals that glow include plutonium (red from heat), radon (yellow to orange to red), and actinium (blue).
- Noble metals, such as silver, gold, and platinum, resist oxidation and corrosion in moist air.
- Precious metals have significant economic importance. Most of the precious metals also are noble metals, since it's important for a currency to resist wear and tear. Examples of precious metals include gold and silver.
- Tungsten is the metal with the highest melting point. Only carbon, a nonmetal, has a higher melting point of all the elements.
- Steel is an alloy made from iron with other metals.
- Bronze is an alloy usually made from copper and tin.
- Brass is an alloy usually made from copper and zinc.
訂閱:
文章 (Atom)